Resources
-
White Paper
Point-of-care testing as a solution for timely early infant diagnosis

Based on the recent encouraging evidence POC/ near-POC EID warrants consideration of rapid adoption and strategic scale up of this solution complementing the existing laboratory network. Ravikiran Bhairavabhotla from CDC briefly outlines the evidence and tools to support the strategic introduction of POC EID as part of a tiered national laboratory network.
Author(s) Ravikiran Bhairavabhotla Originally published on January 17, 2026 Posted on October 12, 2018
-
Publication
A Proposed Framework for the Implementation of Early Infant Diagnosis Point-of-Care

To reduce morbidity and mortality among HIV-infected children and close the treatment gap for HIV-infected children, there is an urgent need to evaluate existing programmatic and laboratory practices for early infant diagnosis and introduce strategies to improve identification of HIV-exposed infants and ensure access to systematic, early HIV testing, with early linkage to treatment for HIV-infected infants. This article describes progress made in follow-up of HIV-exposed infants since 2006, including remaining unmet laboratory and programmatic needs, and recommends strategies for improvement, especially those related to the implementation of point-of-care technology for early infant diagnosis.
Author(s) Diallo et al Originally published on January 1, 2017 Posted on October 12, 2018
-
Guidelines
Early detection of HIV infection in infants and children

Because of the high risk of death before the age of 2 years among HIV-infected infants, and given
the increasing availability of paediatric antiretroviral treatment in many resource-limited settings,
WHO recommends that national programmes should establish the capacity to provide early
virological testing of infants for HIV.Author(s) WHO Originally published on January 17, 2026 Posted on October 12, 2018
-
Guidelines
WHO Information Note: NOVEL POINT-OF-CARE TOOLS FOR EARLY INFANT DIAGNOSIS OF HIV

Novel point-of-care tools for early infant diagnosis of HIV WHO, 2017
Sufficient evidence has been generated on the performance of these assays in the intended field settings to support rapid national regulatory approval and initiation of scale-up. Performance was consistent between laboratory and field settings, and across countries. Further technical evaluations of these technologies are unlikely to add value, but may instead delay implementation and timely diagnoses of HIV-infected infants, a critical and vulnerable population. National regulatory agencies are encouraged to not delay adoption by conducting further evaluations, but instead adopt a rapid and streamlined registration and national approval process for immediate implementation.
Author(s) WHO Originally published on January 1, 2017 Posted on October 12, 2018
-
Other
Point-of-Care Key Considerations and Toolkit Webinar
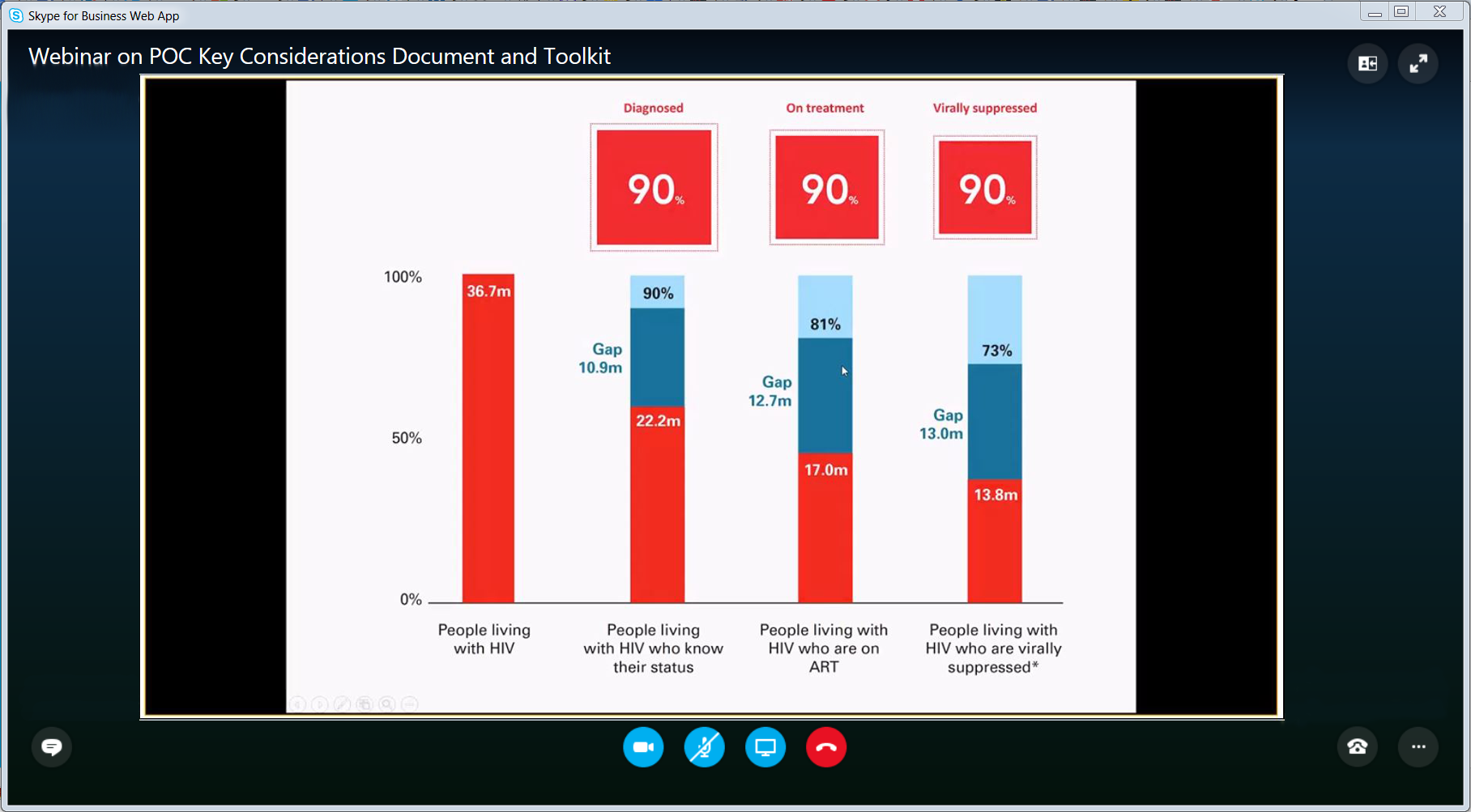
On 15 May, 2018 UNICEF hosted a webinar to provide a broad overview of the Key Considerations Document and the HIV POC Diagnostics Toolkit and to promote coordinated dissemination of both resources amongst partners at the country level. Click the player to watch the webinar.
Author(s) UNICEF Originally published on June 7, 2018 Posted on June 7, 2018
-
Other
Point-of-Care Tool Kit

An extension of Key Considerations for Introducing New HIV Point of Care Diagnostic Technologies in National Health Systems, this Toolkit contains various practical tools and guidance to support countries as they introduce point-of-care (POC) HIV technologies into existing national diagnostic networks and laboratory systems. It provides a roadmap for countries as they seek to expand access to quality-assured HIV diagnostics and work toward achieving the UNAIDS 90-90-90 Fast Track HIV targets.
Author(s) UNICEF Originally published on May 22, 2018 Posted on May 22, 2018
59Resources found...
Filter by ticking the boxes below and then click apply
projects
- SLIPTA
- Laboratory Networks
- LabCoP
- Point-of-Care
- Laboratory Mapping
- Integrated Diagnostic Consortium
- Other
resource type
- Blog Article
- e-Learning
- Guidelines
- Map
- Presentation
- Publication
- Report
- Video
- White Paper
- Other
topics
- Antimicrobial Resistance
- Biosafety/Biosecurity
- Continuous Quality Improvement
- Diagnostic Technology
- Emerging Infectious Diseases
- Global Health Security
- HIV/AIDS
- Laboratory Clinic Interface
- Laboratory Information Systems
- Laboratory System Strengthening
- Laboratory Workforce
- Policy and Guidelines
- Public-Private Partnerships
- Quality Management Systems and Accreditation
- Sample Transportation
- Tuberculosis
- Viral Load Scale-Up
- Waste Management
- Other

