Resources
-
Publication
Report
Guidelines
Safe Management of Wastes from Health Care Activities: A Summary
This document, developed by the World Health Organization (WHO) in 2017, highlights the key aspects of safe healthcare waste management in order to guide policymakers, practitioners and facility managers to improve such services in healthcare facilities. It is based on the comprehensive WHO handbook ‘Safe Management of Wastes from Health-Care Activities’ (WHO, 2014), and also takes into consideration relevant World Health Assembly resolutions, other United Nations documents, and emerging global and national developments on water, sanitation, hygiene, and infection prevention and control.
Author(s) TBD Originally published on January 17, 2026 Posted on February 14, 2019
-
Publication
Report
Guidelines
Waste Issue Resources for Low- and Middle-Income Countries

The waste produced in the course of healthcare activities, from contaminated needles to radioactive isotopes, can cause infection and injury. Inadequate waste management is likely to have serious public health consequences and deleterious effects on the environment.
Author(s) TBD Originally published on January 17, 2026 Posted on February 14, 2019
-
Publication
Viral Load and Early Infant Diagnosis ISME Community of Practice Reference Manual

The VL/EID ISME Reference Manual published by PEPFAR and the Centers for Disease Control and Prevention is a guide for Implementation Subject Matter Experts (ISMEs) who offer either in country or remote technical assistance in VL/EID scale-up.
Author(s) TBD Originally published on January 17, 2026 Posted on February 14, 2019
-
Publication
UNICEF Shares a Mother’s Perspective on Point-of-Care
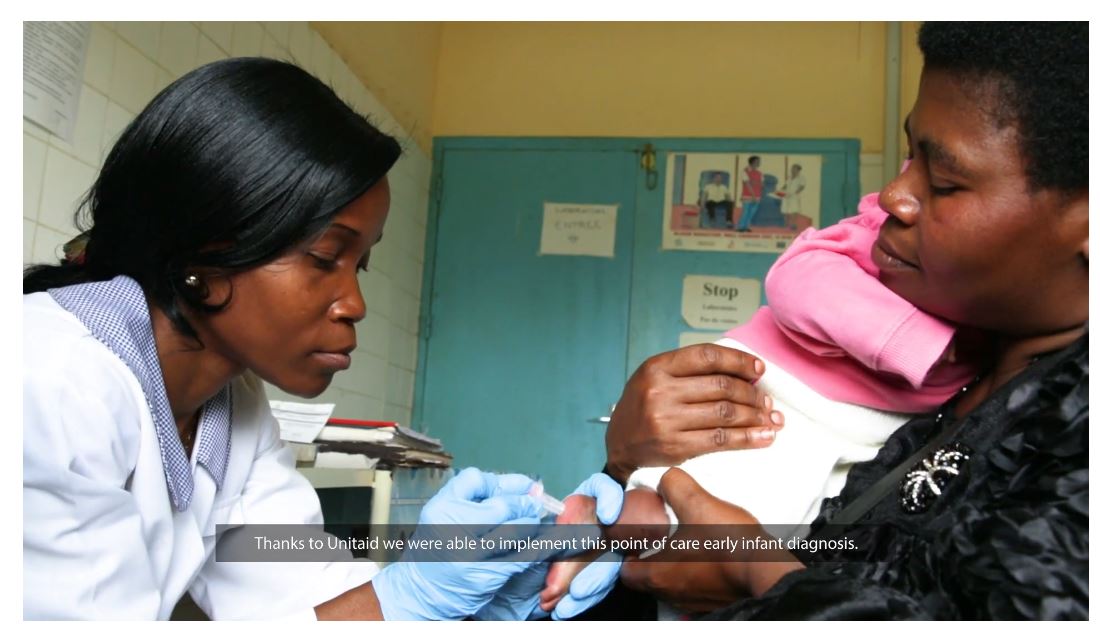
In 2017, UNAIDS estimated 3,300 children under the age of 15 years died from AIDS-related causes in Cameroon. A key challenge is diagnosing and treating infants early in life. Now, a new point-of-care device, which provides results within minutes of testing, has overcome some major obstacles to treatment, giving hope to thousands of mothers and their families.
Author(s) Upjeet Chandan and Karin Schermbrucker (UNICEF) Originally published on December 1, 2018 Posted on December 18, 2018
-
Publication
Developing A Monitoring And Evaluation Framework For Viral Load Testing
The 2013 WHO consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection recommend viral load as the preferred monitoring approach to diagnose and confirm ART failure. As countries invest in the scale-up of routine viral load testing, it is critical to measure the impact and progress towards achieving the UNAIDS target of 90% viral suppression amongst patients on ART by 2020. This document presents key considerations and examples of tools (provided in the appendices) to assist countries in developing a national viral load (VL) monitoring and evaluation (M&E) plan.
Author(s) WHO Originally published on October 1, 2018 Posted on November 15, 2018
-
Publication
What’s New In Treatment Monitoring: Viral Load And CD4 Testing
Treatment initiation
- ART should be initiated in all children, adolescents, pregnant and breastfeeding women and adults living with HIV, regardless of WHO clinical stage and at any CD4 cell count.
- ART should be initiated in all children, adolescents, pregnant and breastfeeding
women and adults living with HIV, regardless of WHO clinical stage and at any CD4 cell count. - As a priority, ART should be initiated in all children, adolescents and adults with severe or advanced HIV clinical disease and adults with a CD4 count ≤ 350 cells/mm3 as well as children < 5 years of age with WHO clinical stage 3 or 4 or CD4 count ≤ 750 cells/mm3.
Author(s) WHO Originally published on July 1, 2017 Posted on November 15, 2018
59Resources found...
Filter by ticking the boxes below and then click apply
projects
- SLIPTA
- Laboratory Networks
- LabCoP
- Point-of-Care
- Laboratory Mapping
- Integrated Diagnostic Consortium
- Other
resource type
- Blog Article
- e-Learning
- Guidelines
- Map
- Presentation
- Publication
- Report
- Video
- White Paper
- Other
topics
- Antimicrobial Resistance
- Biosafety/Biosecurity
- Continuous Quality Improvement
- Diagnostic Technology
- Emerging Infectious Diseases
- Global Health Security
- HIV/AIDS
- Laboratory Clinic Interface
- Laboratory Information Systems
- Laboratory System Strengthening
- Laboratory Workforce
- Policy and Guidelines
- Public-Private Partnerships
- Quality Management Systems and Accreditation
- Sample Transportation
- Tuberculosis
- Viral Load Scale-Up
- Waste Management
- Other

