Resources
-
Guidelines
WHO Information Note: NOVEL POINT-OF-CARE TOOLS FOR EARLY INFANT DIAGNOSIS OF HIV

Novel point-of-care tools for early infant diagnosis of HIV WHO, 2017
Sufficient evidence has been generated on the performance of these assays in the intended field settings to support rapid national regulatory approval and initiation of scale-up. Performance was consistent between laboratory and field settings, and across countries. Further technical evaluations of these technologies are unlikely to add value, but may instead delay implementation and timely diagnoses of HIV-infected infants, a critical and vulnerable population. National regulatory agencies are encouraged to not delay adoption by conducting further evaluations, but instead adopt a rapid and streamlined registration and national approval process for immediate implementation.
Author(s) WHO Originally published on January 1, 2017 Posted on October 12, 2018
-
Other
Point-of-Care Key Considerations and Toolkit Webinar
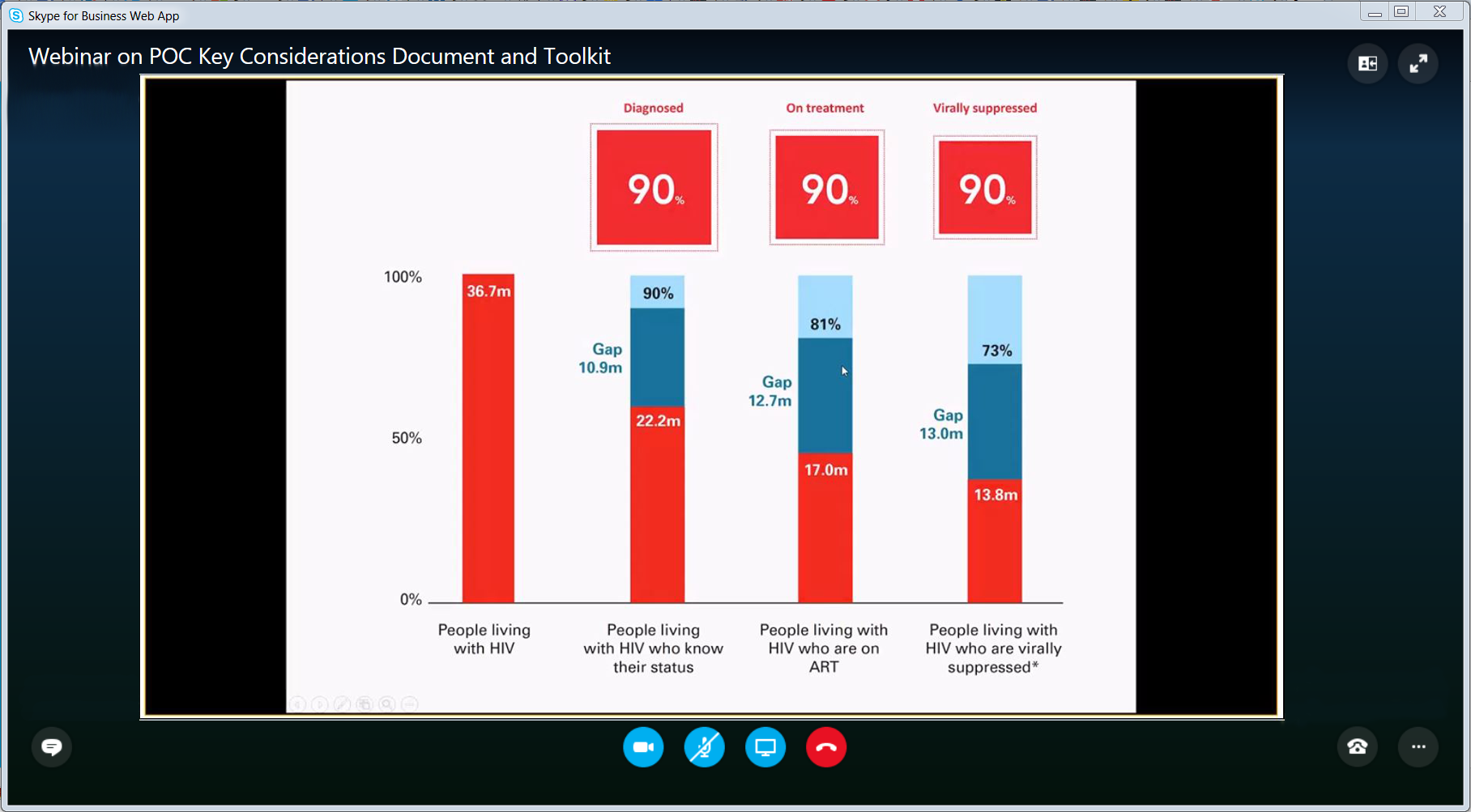
On 15 May, 2018 UNICEF hosted a webinar to provide a broad overview of the Key Considerations Document and the HIV POC Diagnostics Toolkit and to promote coordinated dissemination of both resources amongst partners at the country level. Click the player to watch the webinar.
Author(s) UNICEF Originally published on June 7, 2018 Posted on June 7, 2018
-
Other
Point-of-Care Tool Kit

An extension of Key Considerations for Introducing New HIV Point of Care Diagnostic Technologies in National Health Systems, this Toolkit contains various practical tools and guidance to support countries as they introduce point-of-care (POC) HIV technologies into existing national diagnostic networks and laboratory systems. It provides a roadmap for countries as they seek to expand access to quality-assured HIV diagnostics and work toward achieving the UNAIDS 90-90-90 Fast Track HIV targets.
Author(s) UNICEF Originally published on May 22, 2018 Posted on May 22, 2018
-
Other
Cepheid Receives World Health Organization Pre-Qualification of Xpert HIV-1 Viral Load Test

On July 20, 2017, Cepheid received World Health Organization (WHO) prequalification for its Xpert® HIV-1 Viral Load (VL) test. The Xpert® HIV-1 VL test measures Human Immunodeficiency Virus type 1 (HIV-1) RNA in human plasma from individuals infected with HIV-1 in less than 90 minutes. Measurement of blood plasma HIV-1 RNA concentration (known as HIV viral load) using nucleic acid-based molecular diagnostic assays has been established as the standard of care in assessing HIV-positive patient prognosis and response to antiretroviral therapy. The Xpert® HIV-1 VL test is run on Cepheid’s GeneXpert System, the world’s most prevalent molecular diagnostics platform. By automating highly complex and time-consuming manual procedures, the GeneXpert allows institutions of any size to perform sophisticated genetic testing for organisms and genetic-based diseases.
Author(s) Mr. Nqobile Ndlovu Originally published on August 1, 2017 Posted on January 4, 2018
-
Report
ASLM in New York to support point-of-care device scale-up efforts in Africa

Representatives from the African Society for Laboratory Medicine (ASLM), UNICEF and the Clinton Health Access Initiative, Inc. (CHAI) came together 17-18 August 2017 in New York City, United States, to review progress to date on the implementation of a joint point-of-care project and to refine coordination mechanisms to achieve greater impact.
Author(s) Mr. Nqobile Ndlovu Originally published on September 6, 2017 Posted on January 4, 2018
-
Publication
Pioneering New Diagnostics: Addressing Challenges and Implications for Point-of-Care Testing in African Settings
Point-of-Care Testing: Why is it important?
POCT2Imagine yourself in a city at the centre of one of the world’s deadliest disease outbreaks. You watch as your friends and relatives are struck down by an aggressive illness that kills roughly 50-90% of those affected. One evening, you go to bed tired and achy, awakening the next morning with fever and headache. You do the right thing and call the emergency number. You are told to head to the nearest healthcare facility, a holding centre far from your neighbourhood. You are greeted at the holding centre by security personnel wearing face masks, face shields and gloves. From a distance, they tell you to wash your hands in bleach solution and then point you through a metal door into the facility grounds. A nurse shows you to the “dry” waiting area on the far side of a bright orange plastic fence, an area reserved for suspect cases not yet exhibiting the “wet” symptoms of diarrhoea and vomiting. A few metres away, in another fenced area, you see a chaotic scene: sick people lying on the floor, outnumbered workers in protective suits diligently working to clean diarrhoea and vomit from around them. A nurse in a protective suit approaches you and asks about your symptoms from a distance. She takes your temperature using an infrared device and asks you to wait. Six hours go by and the surveillance team arrives to take a blood sample. Then you wait. Your symptoms worsen the following day. You wait for days at the holding centre. You share bathrooms, eating utensils, and living quarters with up to 100 other sick people. On the third day, your result comes back: you have tested negative for Ebola.
Author(s) Laurel Oldach Originally published on February 1, 2015 Posted on January 4, 2018
38Resources found...
Filter by ticking the boxes below and then click apply
projects
- SLIPTA
- Laboratory Networks
- LabCoP
- Point-of-Care
- Laboratory Mapping
- Integrated Diagnostic Consortium
- Other
resource type
- Blog Article
- e-Learning
- Guidelines
- Map
- Presentation
- Publication
- Report
- Video
- White Paper
- Other
topics
- Antimicrobial Resistance
- Biosafety/Biosecurity
- Continuous Quality Improvement
- Diagnostic Technology
- Emerging Infectious Diseases
- Global Health Security
- HIV/AIDS
- Laboratory Clinic Interface
- Laboratory Information Systems
- Laboratory System Strengthening
- Laboratory Workforce
- Policy and Guidelines
- Public-Private Partnerships
- Quality Management Systems and Accreditation
- Sample Transportation
- Tuberculosis
- Viral Load Scale-Up
- Waste Management
- Other

