Resources
-
Publication
Public-Private Partnership and Strengthening Laboratory Systems in Africa Supplement

This supplement, produced by Labs for Life, a public-private partnership between Becton Dickinson, Company and The President’s Emergency Plan for AIDS Relief (PEPFAR), was published in the Journal of Infectious Diseases. This supplement is designed to support Ministries of Health, Laboratory Experts, Clinical staff, and implementing partners to improve global laboratory systems.
Author(s) TBD Originally published on January 17, 2026 Posted on June 12, 2019
-
Publication
Role of Public-Private Partnerships in Achieving UNAIDS’ 90-90-90 HIV Treatment Targets in Africa

This review article identifies the benefits, enabling factors, and associated challenges for public and private sectors to engage in public-private partnerships. These partnership contributions to laboratory systems strengthening are a model, and present opportunities that can be leveraged to strengthen systems to accelerate the scale up of Viral Load and Early Infant Diagnosis to achieve the UNAIDS 90–90-90 treatment targets for HIV/AIDS.
Author(s) TBD Originally published on June 12, 2019 Posted on June 12, 2019
-
Guidelines
Publication
The Laboratory African Regional Collaborative (LARC) Project Workbook

The Laboratory African Regional Collaborative (LARC) project supports clinicians, laboratorians and data record units to work together and improve the proportion of viral load test results put in patients’ files. LARC is a useful tool that can be used by countries to address inefficiencies at the laboratory-clinical interface at clinic level, and achieve the 3rd ‘90’ of the UNAIDS 90·90-90 HIV targets.
Author(s) TBD Originally published on June 12, 2019 Posted on June 12, 2019
-
Other
HIV Point-of-Care (Nucleic Acid Testing) Landscape in Africa
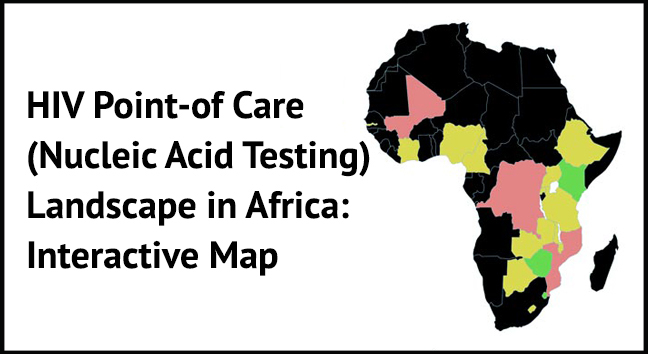
Strategic deployment of POC EID is key to getting more infants tested for HIV and initiated on treatment in a timely manner. This map indicates the distribution by type and numbers of POC Nucleic Acid Testing (NAT) for HIV, EID and VL technologies ( Alere, Samba, GeneXpert, etc. ) being used by some countries in Africa.
Author(s) TBD Originally published on January 17, 2026 Posted on June 10, 2019
-
Other
SLIPTA Audited Laboratories Interactive Distribution Map
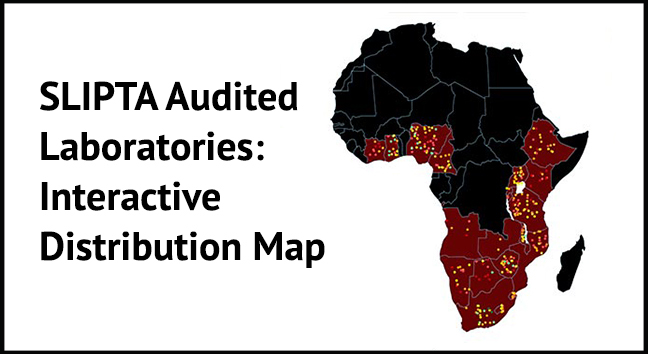
WHO-AFRO has established the Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) programme, a framework for improving the quality of public health laboratories in developing countries to achieve ISO 15189 standards. Through standardised processes, SLIPTA measures and evaluates the progress of laboratory systems towards international accreditation and awards a certificate of recognition (0-5 stars rating). This map shows the distribution and ratings of laboratories that have been audited.
Author(s) TBD Originally published on June 10, 2019 Posted on June 10, 2019
-
Other
Internationally Accredited Laboratories in Africa Map
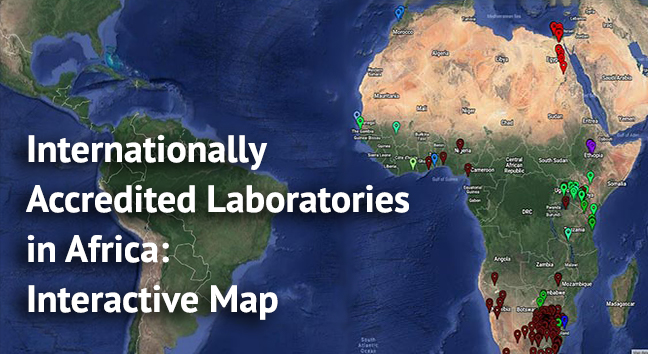
ASLM has created a database of internationally accredited medical laboratories in Africa, plotted on this map. Data were collected from accreditation agencies and on-line sources, as well as through communications with Ministry of Health focal persons, ASLM Ambassadors, and program leaders in each country.
Author(s) TBD Originally published on June 10, 2019 Posted on June 10, 2019
59Resources found...
Filter by ticking the boxes below and then click apply
projects
- SLIPTA
- Laboratory Networks
- LabCoP
- Point-of-Care
- Laboratory Mapping
- Integrated Diagnostic Consortium
- Other
resource type
- Blog Article
- e-Learning
- Guidelines
- Map
- Presentation
- Publication
- Report
- Video
- White Paper
- Other
topics
- Antimicrobial Resistance
- Biosafety/Biosecurity
- Continuous Quality Improvement
- Diagnostic Technology
- Emerging Infectious Diseases
- Global Health Security
- HIV/AIDS
- Laboratory Clinic Interface
- Laboratory Information Systems
- Laboratory System Strengthening
- Laboratory Workforce
- Policy and Guidelines
- Public-Private Partnerships
- Quality Management Systems and Accreditation
- Sample Transportation
- Tuberculosis
- Viral Load Scale-Up
- Waste Management
- Other

