Resources
-
Guidelines
WHO List of Essential Diagnostic Tests to Improve Diagnosis and Treatment Outcomes

WHO has updated its Essential Diagnostics List, a catalogue of the tests needed to diagnose the most common conditions as well as a number of global priority diseases.
Author(s) WHO Originally published on July 31, 2019 Posted on July 31, 2019
-
Guidelines
Publication
Considerations for Adoption and Use of Multi-Disease Testing Devices in Integrated Laboratory Networks

In settings where laboratory testing has been traditionally organized by disease programme, the introduction of multi-disease testing devices brings new opportunities for collaboration and integration. This information note published by WHO provides a strategic overview of key implementation considerations for diagnostic integration of polyvalent testing platforms or multianalyte analysers.
Author(s) TBD Originally published on June 27, 2019 Posted on June 27, 2019
-
Report
Presentation
May 2018 LabCoP ECHO Session Presentation: VL Diagnostic Testing Service Delivery in Kenya

Kenya’s presentation during the May LabCoP ECHO session included their experiences implementing the ‘Plan, Do, Study, Act’ (PDSA) cycle to prioritise and rapidly test ‘change ideas’ needed to facilitate the rapid identification of unsuppressed viral load, followed by swift appropriate clinical action.
Author(s) TBD Originally published on June 18, 2019 Posted on June 18, 2019
-
e-Learning
Video
PEPFAR HIV Rapid Testing Continuous Quality Improvement
This Rapid Testing Continuous Quality Improvement (RTCQI) training video and competency assessment tool is available on a free virtual education platform, PEPconnect to assist ministries of health, health-care providers, and stakeholders in planning, implementing and sustaining quality assurance for point-of-care testing.
Author(s) TBD Originally published on June 18, 2019 Posted on June 18, 2019
-
Video
Early Infant Diagnosis Through Dried Blood Spot Collection
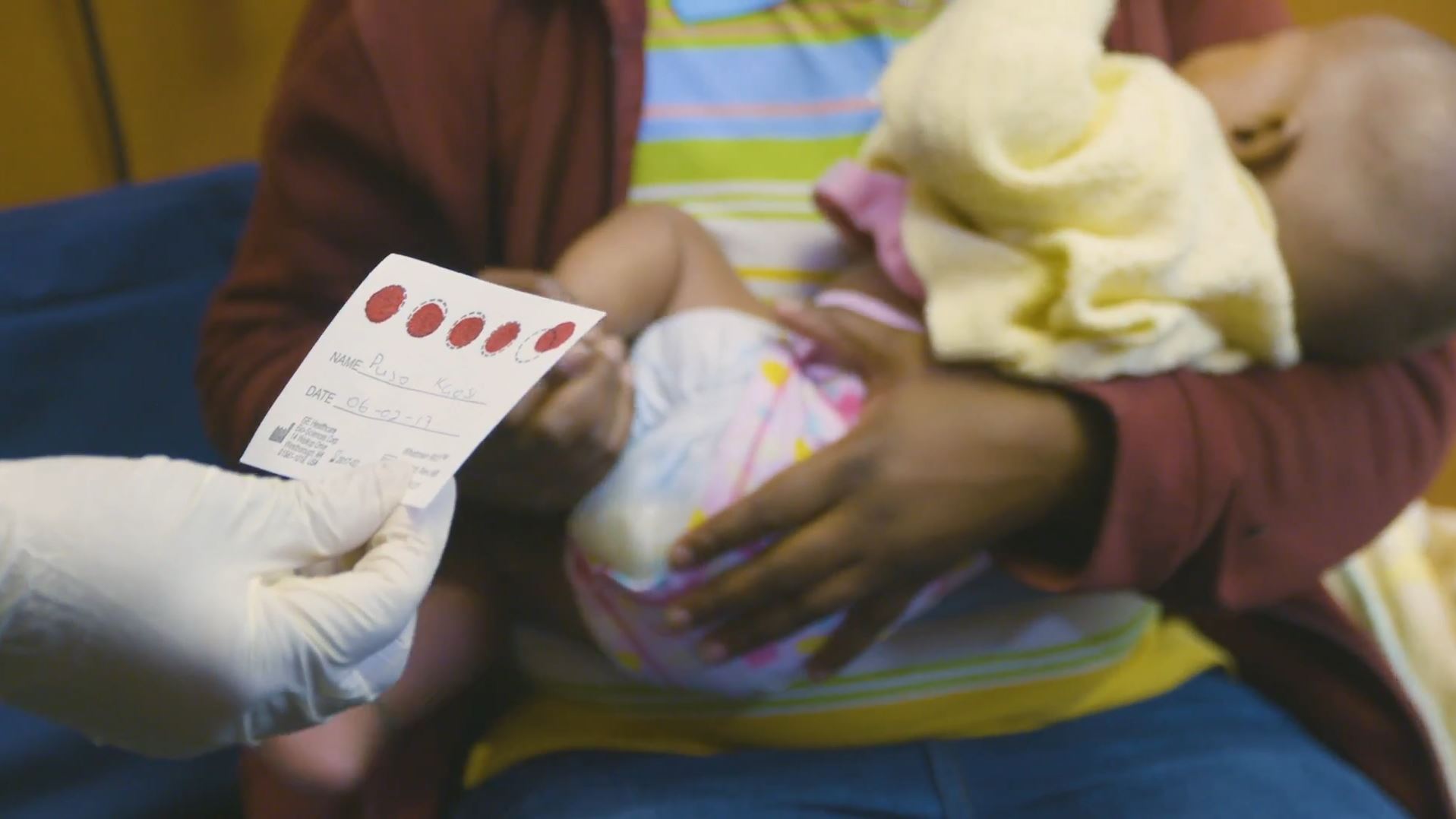
This video, produced by the US Centers for Disease Control and Prevention in English, French, and Portuguese, describes the procedure for collection of a dried blood spot (DBS) specimen from an infant below 18 months of age for performing HIV-1 PCR. Early infant diagnosis (EID) is critical for timely initiation of antiretroviral treatment (ART) in HIV-infected children who are at high risk of mortality.
Author(s) TBD Originally published on December 27, 2025 Posted on June 17, 2019
-
Video
Collection of Dried Blood Spot Specimen for Viral Load Testing
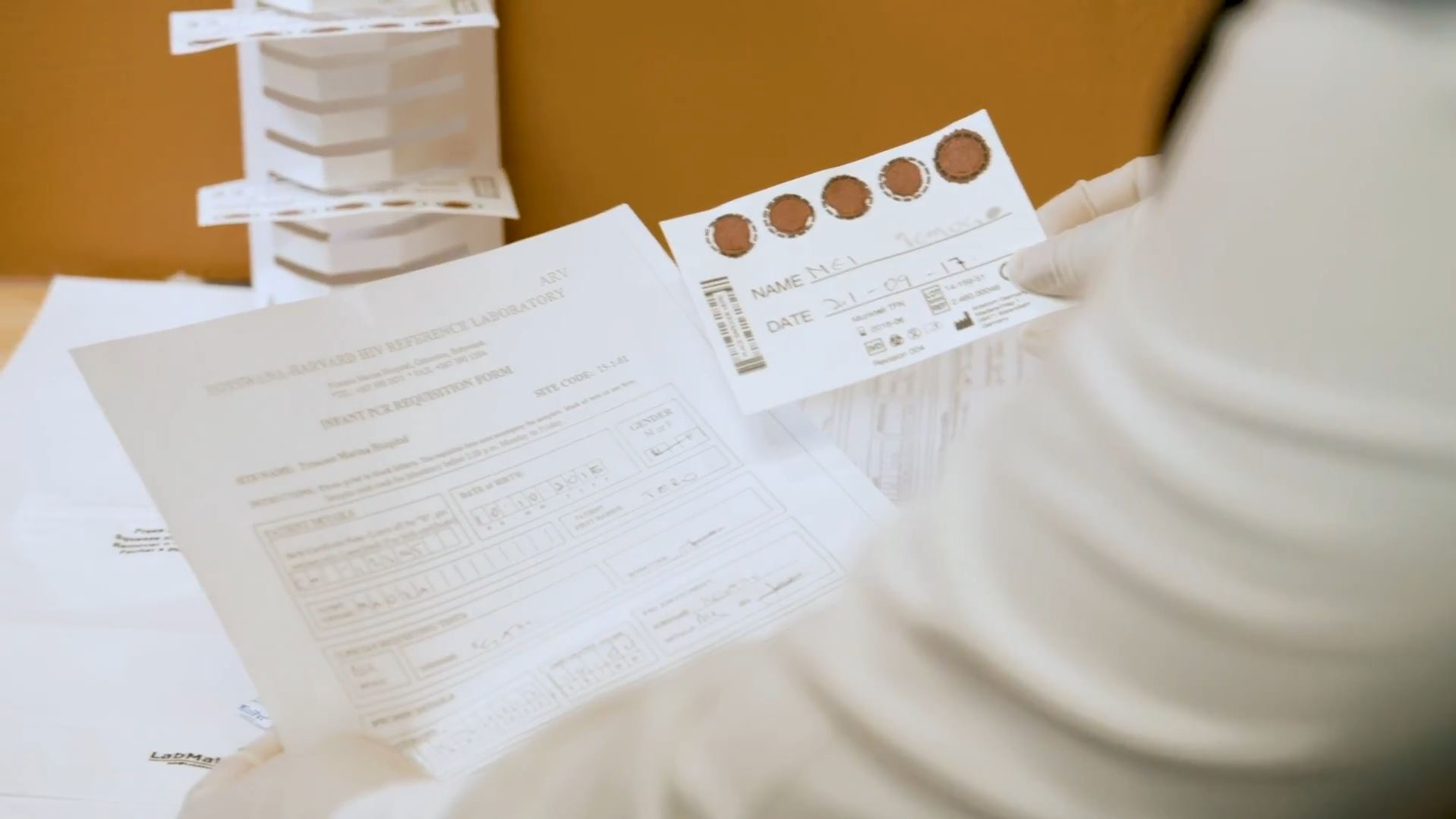
In 2013, the World Health Organization recommended viral load (VL) monitoring as the gold standard for monitoring the effectiveness of antiviral therapy (ART). This video, produced by the US Centers for Disease Control and Prevention in English, French, and Portuguese, shows the recommended step-by-step process for the collection of dried blood spot (DBS) samples for quality assured VL results.
Author(s) TBD Originally published on June 17, 2019 Posted on June 17, 2019
38Resources found...
Filter by ticking the boxes below and then click apply
projects
- SLIPTA
- Laboratory Networks
- LabCoP
- Point-of-Care
- Laboratory Mapping
- Integrated Diagnostic Consortium
- Other
resource type
- Blog Article
- e-Learning
- Guidelines
- Map
- Presentation
- Publication
- Report
- Video
- White Paper
- Other
topics
- Antimicrobial Resistance
- Biosafety/Biosecurity
- Continuous Quality Improvement
- Diagnostic Technology
- Emerging Infectious Diseases
- Global Health Security
- HIV/AIDS
- Laboratory Clinic Interface
- Laboratory Information Systems
- Laboratory System Strengthening
- Laboratory Workforce
- Policy and Guidelines
- Public-Private Partnerships
- Quality Management Systems and Accreditation
- Sample Transportation
- Tuberculosis
- Viral Load Scale-Up
- Waste Management
- Other

