Resources
-
Guidelines
Publication
White Paper
Integrated Testing for TB and HIV Using GeneXpert Devices Expands Access to near Point-Of-Care Testing

This brief summarizes the key findings and lessons learned from Zimbabwe’s pilot implementation of TB/HIV testing integration, while also highlighting the benefits of integrated testing for clients, health providers and the health system.
Author(s) TBD Originally published on September 4, 2019 Posted on September 4, 2019
-
e-Learning
Guidelines
Presentation
Video
WHO Biosafety Video Series – Good Microbiological Practice and Procedures

WHO has produced a biosafety video series entitled, “Good Microbiological Practice and Procedures (GMPP)”, which is central to the WHO Laboratory Biosafety Manual (LBM4) being revised and finalised. These seven training videos will help enhance the safety of any health laboratory, including those in resource-limited settings.
Author(s) TBD Originally published on September 4, 2019 Posted on September 4, 2019
-
Guidelines
Publication
White Paper
Accelerating Access to Point-of-Care Viral Load Testing for Pregnant and Breastfeeding Women Living with HIV

This issue brief lays out the rationale for how Point-of-Care Viral Load (POC VL) testing could be a transformative technology in the strategy to end vertical transmission. It was developed by global health partners ASLM, CHAI, UNICEF and Unitaid, and is available in both French and English
Author(s) TBD Originally published on September 2, 2019 Posted on September 4, 2019
-
Publication
Report
Latest Data and WHO HIV Policy Adoption and Implementation Status in Countries
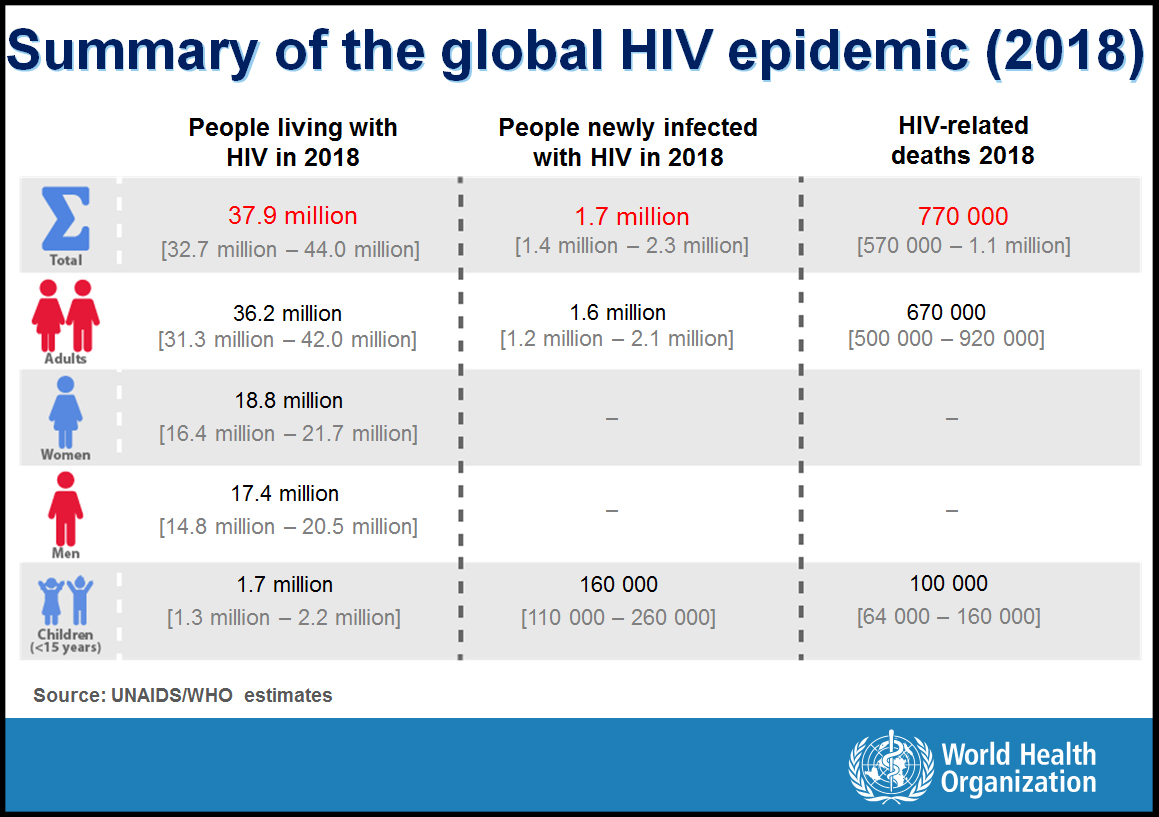
Herein, data are presented for 194 WHO Member States – including 137 low- and middle-income countries, and 35 Fast-Track countries, respectively, through July 2019. The HIV fact sheet by WHO region, policy maps, and key data for HIV have been updated with the latest release of data for 2018 during the last week of July 2019.
Author(s) TBD Originally published on September 4, 2019 Posted on September 4, 2019
-
Guidelines
Publication
Report
White Paper
HIV Molecular Diagnostics Toolkit to Improve Access to Viral Load Testing and Infant Diagnosis

The HIV Molecular Diagnostics Toolkit to Improve Access to Viral Load Testing and Infant Diagnosis, published by WHO, presents new and tried concepts for scaling up HIV VL testing across settings.
Author(s) TBD Originally published on August 30, 2019 Posted on August 30, 2019
-
Guidelines
LabCoP VL Cascade and Theory of Action

Embracing the intricate context of the viral load testing continuum, how it contributes to better patient management, how it relates to the underlying laboratory systems, and how it involves national stakeholders, LabCoP aims to help improve laboratory system functions and accelerate the scale-up of VL for improved patient management, according to this theory of action infographic.
Author(s) TBD Originally published on August 27, 2019 Posted on August 27, 2019
59Resources found...
Filter by ticking the boxes below and then click apply
projects
- SLIPTA
- Laboratory Networks
- LabCoP
- Point-of-Care
- Laboratory Mapping
- Integrated Diagnostic Consortium
- Other
resource type
- Blog Article
- e-Learning
- Guidelines
- Map
- Presentation
- Publication
- Report
- Video
- White Paper
- Other
topics
- Antimicrobial Resistance
- Biosafety/Biosecurity
- Continuous Quality Improvement
- Diagnostic Technology
- Emerging Infectious Diseases
- Global Health Security
- HIV/AIDS
- Laboratory Clinic Interface
- Laboratory Information Systems
- Laboratory System Strengthening
- Laboratory Workforce
- Policy and Guidelines
- Public-Private Partnerships
- Quality Management Systems and Accreditation
- Sample Transportation
- Tuberculosis
- Viral Load Scale-Up
- Waste Management
- Other

