The World Health Organization’s Regional Office for Africa (WHO AFRO) has established a framework for improving the quality of public health laboratories in developing countries to achieve ISO 15189 standards. This framework, implemented by ASLM in Africa, is the Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) programme.
The World Health Organization’s Regional Office for Africa (WHO AFRO) has established a framework for improving the quality of public health laboratories in developing countries to achieve ISO 15189 standards. This framework, implemented by ASLM in Africa, is the Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) programme.
Through standardised processes, SLIPTA measures and evaluates the progress of laboratory systems towards international accreditation and awards a certificate of recognition (0-5 star ratings). SLIPTA enables laboratories to develop their quality management systems in order to produce timely, reliable and accurate laboratory results.
What is the difference between SLIPTA and SLMTA? Click here.
Read the latest SLIPTA Audited Laboratories Distribution Map
The WHO Guide for the Stepwise Laboratory Improvement Process Towards Accreditation in the African Region (with checklist).
- Lignes Directrices de l’OMS Relatives au Processus Graduel a’Amelioration des Laboratoires en Vue de l’accreditation (SLIPTA) dans la Region Africaine (accompagnées d’une liste de contrôle)
- Orientações da OMS para o Processo Gradual de Melhoria Laboratorial com Vista à Acreditação na Região Africana (com lista de verificação)
Laboratory Accreditation Updates
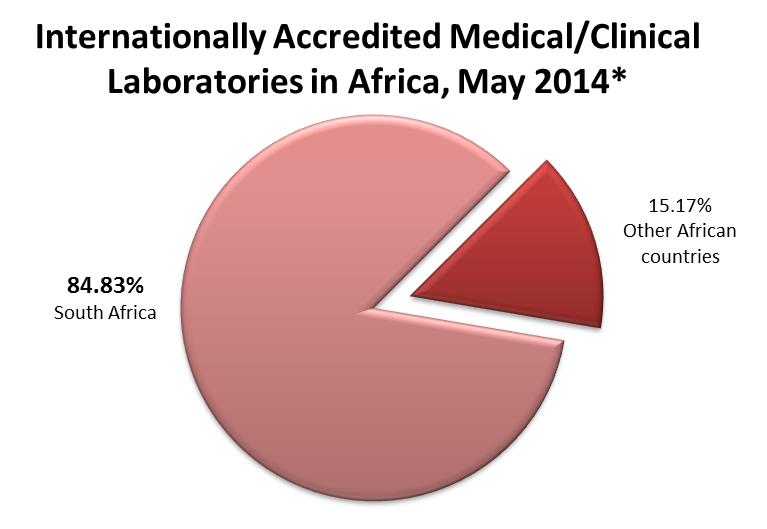
*ASLM has gathered publicly available data from the following (retrieved May 2014):
-
-
- College of American Pathologists (CAP)
- Comite Francais d’Accreditation (COFRAC)
- Egyptian Accreditation Council (EGAC)
- Ethiopian National Accreditation Office (ENAO)
- Joint Commission International (JCI)
- Kenya Accreditation Service (KENAS)
- South African National Accreditation System (SANAS)
- Southern African Development Community Accreditation Service (SADCAS)
- Swedish Board for Accreditation and Conformity Assessment (SWEDAC)
-
To contribute to, or provide feedback on the compilation of the above laboratory accreditation data, or to learn more about ASLM SLIPTA implementation please send us an SLIPTA@http://localhost.